This nonfiction article is written for use with upper-elementary students (grades 4-5). Students learn about the molecular structure of water, why water expands as it freezes, and why ice’s ability to float is essential to life on Earth. The concepts and text structure of this article are challenging, and we recommend using the related activities (see below) to support student comprehension.
Modified versions are available for students in grades K-1 and grades 2-3, or any student needing a simplified version. Students in grades K-1 explore the many forms of floating ice on Earth. Students in grades 2-3 are introduced in a simplified manner to the concepts that water expands as it freezes and floats. As always, consider the reading level and needs of your students when selecting a version for classroom use.
At each grade level, the article is available in three forms. Printable pdf files allow you to print this story in either text or a foldable book format. Your students can listen to the story while they read our electronic book version. Related activities provide tips for integrating this story with your science and literacy instruction.
Interested in other nonfiction articles for your students? Browse all twenty sets from the Beyond Penguins and Polar Bears collection on our Stories for Students page!
 Growing Floaters and Shrinking Sinkers
Growing Floaters and Shrinking Sinkers
Flesch-Kincaid Reading Level = 5.1
Grab an ice cube from the freezer and drop it into your favorite after-school drink. What happens? You probably saw your cube sink a bit, then bob up to the surface and just stay there, floating like a cork or a bath toy. Big deal, right? Wrong! What you’ve just experienced is one of the oddest and most important events on Earth. Ice floats in water, and that makes water weird!
Ice is of course just solid water. Hold an ice cube in your hand and you quickly get a wet hand, because your body heat changes the ice from a solid to a liquid. Heat changes other substances (like iron, for instance) from solid to liquid, too. But there’s a big difference.
For most substances, the solid form sinks in the liquid form. Imagine a vat of molten iron. Toss in a chunk of solid iron, and it sinks like a stone. Solid iron sinks in molten iron because as solid iron forms, it shrinks.
Shrinking Iron
Think about what really happens when something shrinks. Everything around us is made of atoms. The atoms that make up molten iron are moving fast! They are bouncing off each other at high speed. As the molten iron cools, the atoms slow down a little. The slower atoms don’t bounce off each other quite so forcefully, and so the atoms end up closer together. Eventually, the atoms are so slow that they get locked into place, in a shape called a crystal.
You might think of crystals as beautiful, shiny jewels. When scientists talk about crystals, though, they’re talking about something else. A crystal is just an ordered arrangement of atoms. When molten iron cools, it forms crystals. If it cools slowly, it forms one large crystal. If it cools quickly, it forms lots of little crystals. Either way, the atoms in the crystal solid are closer together than the atoms in the liquid. This is why the solid iron sinks in the molten iron.
That’s true for almost every material you can think of. The atoms in the solid are closer together than in the liquid. The solid shrinks, and it sinks in the liquid. But that’s not true for water. Something weird happens as water gets colder and changes to ice.
Growing Ice
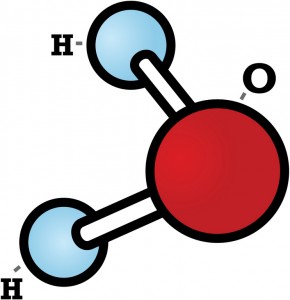
But what happens when water turns to ice? The best way to understand is to build your own water molecule. To build this model, you’ll need gumdrops, small marshmallows, and toothpicks.
Every water molecule is made of one atom of oxygen and two atoms of hydrogen. Let’s use gumdrops for the oxygen atoms. The marshmallows will be the hydrogen atoms. The toothpicks will hold the atoms together.
First stick two toothpicks into a gumdrop. But don’t stick the toothpicks straight across from one another! A water molecule is bent a little. It looks something like this:
Let’s think about that liquid water for a minute. Imagine a whole sea of the water molecules floating past one another. Their shape lets them get pretty close to each other without touching. As the temperature drops, they move slower and slower. The slower they move, the closer they can get to each other.
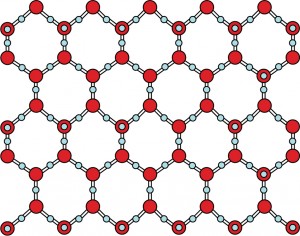
That’s when something strange happens. Just like cooling iron, the cooling water starts to form crystals. But these crystals have a very special shape.
Why? It has to do with the atoms. It turns out the hydrogen atoms can get close to oxygen atoms. But they can’t get close to other hydrogen atoms. In your model, this means that the marshmallows can get close to the gumdrops, but not to the other marshmallows. This causes the water molecules to line up something like this:
In this picture, each blue dot is an oxygen atom, or a gumdrop. Each red dot is a hydrogen atom, or a marshmallow. Do you notice how much space there is between the water molecules? That extra space is what makes ice float!
When water cools down, its molecules get closer together. The molecules start to form these wide-open crystals. More and more crystals form until the water has turned to ice. The wide-open crystal structure means that the freezing water didn’t shrink. It grew!
Pop, Potholes, and Polar Bears
If you’ve ever left a bottle or can of pop in a freezer, you know how powerful this growing ice can be. It can even break glass! The ice inside pushes harder and harder on the container’s walls until the container gives way, sometimes in an explosion. The same thing makes potholes in roads. First, water seeps into small cracks in the road. Then the water freezes. The freezing water grows, and the cracks get bigger. More water creeps in, freezes, and grows, starting the whole thing all over again.
Growing water isn’t all bad, however. In fact, without water’s weird way of growing as it freezes, the world would be a very different place. Imagine if ice sank to the bottom of lakes, or even the ocean. Once there, the ice would likely never melt again. Much of the world’s water would be trapped forever far below the surface of lakes and the ocean.
If lakes froze from the bottom up, fish could never survive the winter. And what if ice didn’t float on seawater? Polar bears, seals, and many other creatures would need to find a new way of life. They depend on the floating ice of the Arctic.
The next time you cool off your favorite drink with a bit of solid water, consider what an amazing event you’ve just witnessed. Ice floats, and that’s weird!
Glossary
atom – small particles that make up everything around us
molecule – several atoms joined together
molten – melted or liquid
Modified versions of this text are available for grades K-1 (Flesch-Kincaid Reading Level = 1.4) and grades 2-3 (Flesch-Kincaid Reading Level = 3.0). See below for links to all three versions in text, book, and electronic book forms.
Printable Files
| Print the text-only version of this article for grades: | |||
| Print book versions of this article for grades: |
Notes for assembling the books:
You can put this book together a couple of different ways. You can print out the pages, cut them in half and then order the pages back to front. Fold the stack in half and then staple the spine of the book. Pairs of pages can then be stapled or glued along the right edge.
You can also assemble the book as a foldable book.
To assemble the books this way, print the four pages and align the document pages so that the following book page numbers are in the lower right-hand corner: front page, page 6, page 2, and page 4. (The cover page should be on top and page 4 on the bottom.) Set your copier to copy single pages into double pages and run the four document pages in the order specified. Cut along the dotted line in the center of the double-sided page, place the book pages in order, fold, and staple along the spine.
Electronic Books
Growing Floaters and Shrinking Sinkers
Grades K-1 Electronic Book
Articulate Version
Flash Version
Grades 2-3 Electronic Book
Articulate Version
Flash Version
Grades 4-5 Electronic Book
Articulate Version
Flash Version
In the Articulate version, click on the small arrow at the top of each page for the narration. The large arrow at the right will take you to the next page.
In the Flash version, the play button (in the top right hand corner) will play an audio file of the text on that page, while the pawprint (bottom right hand corner) will turn to the next page. Please note that the audio files take a moment to load on each page. Once the file has been loaded, a play button will appear in the top right hand corner of the page. To minimize the delay on each page, you can open the file and read through the article first. Once each page’s audio has loaded, it remains loaded until you close the browser window. By preparing the article ahead of time, you can have students start at the beginning of the book and read without delays. If you don’t have Flash, you can download it for free from the Adobe web site.
We’ve also created a literacy set that includes all of the illustrated and electronic books in one convenient location.
Related Activities
These lessons and activities can help you integrate this article into your science and literacy instruction. The activity suggested in the story (creating a molecular model of water) will help students in grades 4 and 5 visualize why water expands as it freezes. For younger students, firsthand observation of water freezing and melting is sufficient. Several activities allow students to investigate the consequences of climate change and melting ice in terms of sea level.
Sea Ice Clip Set (Grades K-5)
This Content Clips set includes 11 images of various forms of ice and snow found in the polar regions and a video (from the Teacher’s Domain collection) about sea ice.
Water and Ice (Grades K-2)
Students use observation, measurement, and communication skills to describe what happens to water as it goes from solid to liquid and back again.
Amazing Ice Cubes: Floating and Sinking (Grades 3-5)
Unlike nearly all other substances, water expands when it freezes, and shrinks when it melts. Students discover this unusual property by observing the mesmerizing process of an ice cube melting in cooking oil. A teacher demonstration of water contracting as it melts is prepared at the beginning of the lesson and discussed after the student investigations.
When Floating Ice Melts in the Sea (Grades 3-5)
Students use water and ice cubes to model what happens when floating ice melts. Students (and teachers) will observe that the melting of floating ice, such as icebergs and ice shelves, does not affect sea level. It may be a good idea to revise the procedures to include something to catch the overflow of water. Challenge your students to modify the experiment to show what happens to land masses surrounded by water when ice melts.
When Land Ice Melts (Grades 3-5)
Students model the melting of land ice (glaciers and ice sheets) to discover that this type of melting does affect sea level. As with the experiment involving floating ice, it is a good idea to include something to catch the overflow of water. Challenge students to think about the block of wood. Does this effectively model what would happen to the land? What does the water represent? How could they modify the procedure to investigate what happens to another body of land in the same ocean?
This article was written by Stephen Whitt. For more information, see the Contributors page. Email Kimberly Lightle, Principal Investigator, with any questions about the content of this site. The content of this page was updated in June 2020.
Copyright August 2008 – The Ohio State University. This material is based upon work supported by the National Science Foundation under Grant No. 0733024. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This work is licensed under an Attribution-ShareAlike 3.0 Unported Creative Commons license.